Myelodysplastic Syndromes
Summary
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal haematopoietic stem cell disorders characterised by ineffective haematopoiesis, peripheral cytopenias, dysplastic morphology, and risk of transformation to acute myeloid leukaemia (AML). MDS predominantly affects older adults (median age 70) and presents with symptoms of bone marrow failure: anaemia (fatigue), neutropenia (infections), and thrombocytopenia (bleeding). Diagnosis requires bone marrow examination showing dysplasia in ≥10% of cells in one or more lineages. Risk stratification using the Revised International Prognostic Scoring System (IPSS-R) is essential for guiding treatment: lower-risk MDS is managed with supportive care and erythropoiesis-stimulating agents (ESAs), while higher-risk MDS requires disease-modifying therapy such as azacitidine or allogeneic stem cell transplantation.
Key Facts
- Definition: Clonal haematopoietic stem cell disorder with dysplasia, ineffective haematopoiesis, and risk of AML transformation
- Incidence: 3-5 per 100,000; rises to >20 per 100,000 in over 70s
- Peak Demographics: Elderly, median age 70; M > F slightly
- AML Transformation: 30% overall (higher in high-risk subtypes)
- Gold Standard Investigation: Bone marrow aspirate and trephine with cytogenetics
- Risk Stratification: IPSS-R (Very Low to Very High)
- First-line Treatment: Low-risk: supportive care, ESAs; High-risk: azacitidine, allo-SCT
- Prognosis: Median survival ranges from 8 years (very low risk) to less than 1 year (very high risk)
Clinical Pearls
Diagnostic Pearl: MDS is a diagnosis requiring bone marrow examination - peripheral blood alone is insufficient. Look for dysplasia in ≥10% of cells.
Treatment Pearl: Erythropoietin levels help predict response to ESAs - levels less than 500 mU/mL associated with better response.
Pitfall Warning: Exclude B12/folate deficiency and reactive causes of dysplasia before diagnosing MDS. Rule out nutritional causes first.
Mnemonic: MDS - Morphology (dysplasia), Dysplasia in marrow, Stem cell clonal disorder
Why This Matters Clinically
MDS is increasingly common with ageing populations. Risk stratification fundamentally changes management intensity. Distinguishing from reactive causes of cytopenia is essential. Allogeneic transplant is potentially curative in eligible patients.
Incidence
- Overall: 3-5 per 100,000 per year
-
70 years: >20 per 100,000 per year
- Increasing incidence due to ageing population and increased recognition
Demographics
| Factor | Details |
|---|---|
| Age | Median 70 years; rare less than 50 |
| Sex | M:F 1.5:1 |
| Ethnicity | Similar across ethnicities |
Risk Factors
| Factor | Mechanism |
|---|---|
| Age | Accumulation of somatic mutations |
| Prior chemotherapy | Therapy-related MDS (alkylators, topoisomerase inhibitors) |
| Prior radiotherapy | DNA damage |
| Benzene exposure | Occupational exposure |
| Smoking | Increased risk |
| Congenital syndromes | Fanconi anaemia, Shwachman-Diamond |
Mechanism
Step 1: Initiating Mutations
- Somatic mutations in haematopoietic stem cells
- Common genes: SF3B1, TET2, ASXL1, DNMT3A, TP53, RUNX1
- Clonal expansion of mutated cells
Step 2: Dysplastic Haematopoiesis
- Altered differentiation and maturation
- Morphological dysplasia in erythroid, myeloid, megakaryocytic lineages
- Increased apoptosis in marrow (ineffective haematopoiesis)
Step 3: Peripheral Cytopenias
- Despite cellular marrow, peripheral blood shows cytopenias
- Anaemia most common; also neutropenia, thrombocytopenia
- Functional defects in mature cells
Step 4: Clonal Evolution
- Acquisition of additional mutations
- Increasing blast percentage
- Transformation to AML (≥20% blasts)
Step 5: Disease Progression
- Without intervention: progressive marrow failure or AML
- Treatment may delay progression
Classification (WHO 2022)
| Subtype | Dysplasia | Blasts (BM) | Key Feature |
|---|---|---|---|
| MDS-SLD | Single lineage | less than 5% | Low risk |
| MDS-MLD | 2-3 lineages | less than 5% | |
| MDS-RS | Ring sideroblasts ≥15% | less than 5% | SF3B1 mutation |
| MDS-EB-1 | Any | 5-9% | Higher risk |
| MDS-EB-2 | Any | 10-19% | High risk, near AML |
| MDS with del(5q) | Variable | less than 5% | Good prognosis, Lenalidomide |
| MDS-U | Unclassifiable | less than 5% |
Symptoms
Anaemia (most common):
Neutropenia:
Thrombocytopenia:
Signs
Red Flags
[!CAUTION]
- Severe symptomatic anaemia (Hb less than 70 g/L)
- Neutropenic fever
- Major bleeding
- Rapidly increasing blasts (transformation)
Assessment
General:
- Pallor, cachexia
- Petechiae, bruising
Abdominal:
- Splenomegaly (mild, if present)
Lymphadenopathy:
- Typically absent (if present, consider alternative diagnosis)
Signs of infection:
- Mouth ulcers
- Skin infections
First-Line Blood Tests
| Test | Finding |
|---|---|
| FBC | Cytopenia(s): anaemia, neutropenia, thrombocytopenia |
| Blood film | Dysplastic changes, macrocytosis, hypogranular neutrophils |
| Reticulocytes | Low (ineffective erythropoiesis) |
| B12, Folate | Normal (exclude deficiency) |
| LDH | May be elevated |
| Ferritin | May be elevated (transfusion iron loading) |
Bone Marrow Examination (Essential)
| Component | Purpose |
|---|---|
| Aspirate | Morphology, blast count, ring sideroblasts |
| Trephine | Cellularity, fibrosis |
| Cytogenetics | Karyotype (prognostic) |
| FISH | del(5q), monosomy 7, etc. |
| Molecular | NGS panel (SF3B1, TP53, ASXL1, etc.) |
| Iron stain | Ring sideroblasts |
Risk Stratification (IPSS-R)
| Risk Group | Median Survival | 25% AML Transformation |
|---|---|---|
| Very Low | 8.8 years | Not reached |
| Low | 5.3 years | 10.8 years |
| Intermediate | 3 years | 3.2 years |
| High | 1.6 years | 1.4 years |
| Very High | 0.8 years | 0.7 years |
Components: Cytogenetics (very good → very poor), blasts %, Hb, platelets, neutrophils
Algorithm
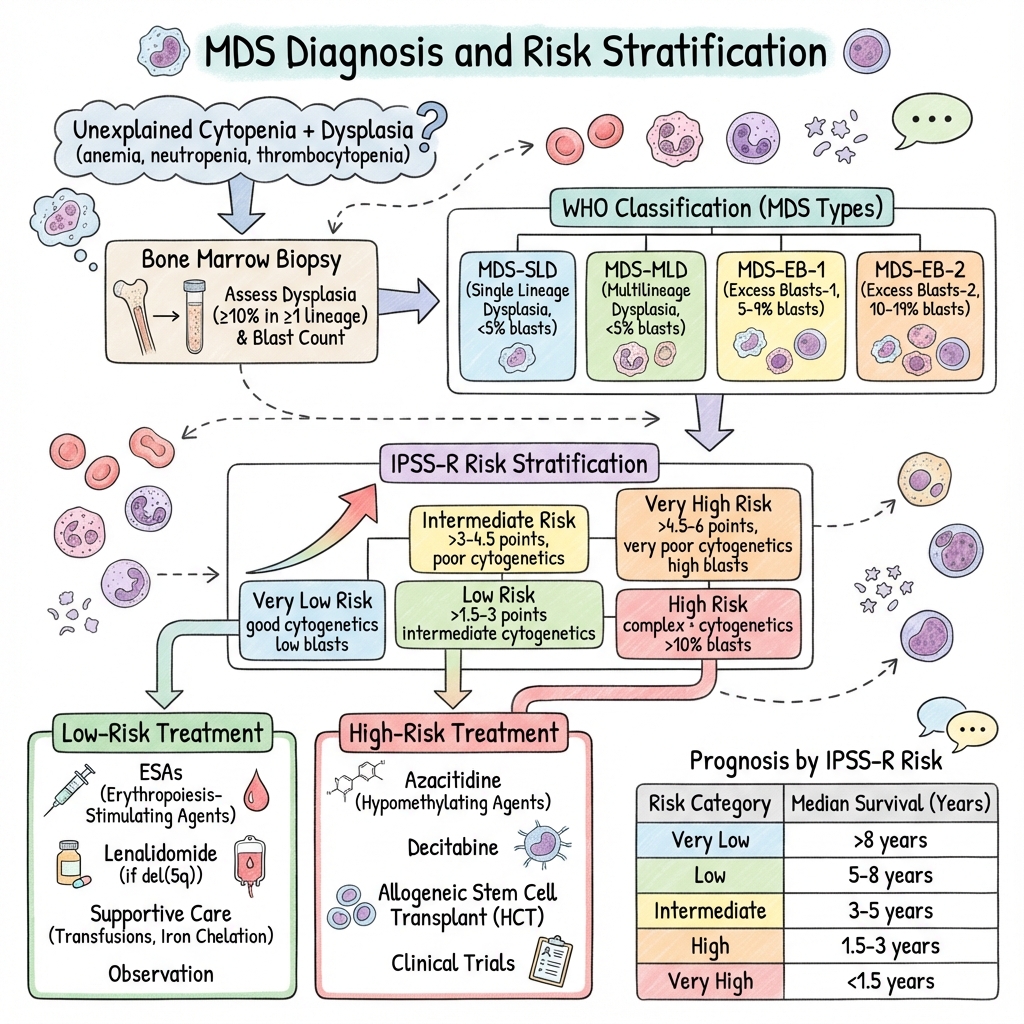
Risk-Stratified Treatment
Lower-Risk MDS (IPSS-R Very Low/Low/Intermediate-1):
| Intervention | Indication |
|---|---|
| Watchful waiting | Asymptomatic, stable |
| ESAs (Epoietin/Darbepoetin) | Symptomatic anaemia, EPO less than 500, transfusion-independent potential |
| Lenalidomide | del(5q) MDS |
| Luspatercept | Ring sideroblasts, ESA failure |
| Transfusion support | Symptomatic anaemia |
| Iron chelation | Transfusion-dependent, >20 units |
Higher-Risk MDS (IPSS-R Intermediate-2/High/Very High):
| Intervention | Indication |
|---|---|
| Azacitidine | Standard disease-modifying therapy; 75mg/m² SC days 1-7, 28-day cycles |
| Allogeneic SCT | Only curative option; consider in fit patients |
| Intensive chemotherapy | AML-like regimens if transformation imminent or allo-SCT planned |
| Clinical trials | Consider enrollment |
Supportive Care
- Transfusions: Red cells, platelets as needed
- Infection prophylaxis: Consider if neutropenic
- Iron chelation: Deferasirox for iron overload (ferritin >1000)
- G-CSF: Selected cases with recurrent infections
Special Considerations
Allogeneic SCT:
- Only curative option
- Consider in less than 70 years, fit, with donor
- Optimal timing debated (transplant earlier vs. wait for progression)
TP53-mutated MDS:
- Very poor prognosis
- Consider clinical trials
- Transplant outcomes poor
| Complication | Incidence | Management |
|---|---|---|
| AML transformation | 30% | Intensive treatment, allo-SCT |
| Infection (neutropenia) | Common | Antibiotics, G-CSF |
| Bleeding | Common | Platelet transfusion |
| Iron overload | Transfusion-dependent | Chelation |
| Treatment-related mortality | Variable | Careful patient selection |
Outcomes by IPSS-R
| Risk | Median Survival |
|---|---|
| Very Low | 8.8 years |
| Low | 5.3 years |
| Intermediate | 3 years |
| High | 1.6 years |
| Very High | 0.8 years |
Prognostic Factors
Favourable:
- del(5q) isolated
- SF3B1 mutation
- Lower blast percentage
- Younger age
Unfavourable:
- TP53 mutation
- Complex karyotype
- High blast percentage
- Therapy-related MDS
Key Guidelines
- NCCN Guidelines: MDS (2024) — Comprehensive US guidance
- ELN Recommendations (2022) — European consensus. PMID: 34773327
- BSH Guidelines (2023) — UK management recommendations
Key Trials
CALGB 9221 — Azacitidine vs supportive care; improved survival in higher-risk MDS. PMID: 15269313
Lenalidomide in del(5q) — High response rates. PMID: 16929061
What is MDS?
MDS is a group of blood disorders where your bone marrow doesn't make healthy blood cells properly. This leads to low blood counts - anaemia (low red cells), infection risk (low white cells), and bleeding risk (low platelets).
What causes it?
In most people, there's no clear cause. It happens more often in older adults. Previous chemotherapy or radiation treatment can increase risk.
How is it treated?
- Blood transfusions for anaemia
- Medications to boost blood cell production
- Injections to help make more red cells
- Chemotherapy for higher-risk disease
- Some people may be suitable for a stem cell transplant
-
Greenberg PL et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes (IPSS-R). Blood. 2012;120(12):2454-2465. PMID: 22740453
-
Arber DA et al. The 2016 revision to the WHO classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. PMID: 27069254
-
Fenaux P et al. Efficacy of azacitidine compared with conventional care regimens (AZA-001). Lancet Oncol. 2009;10(3):223-232. PMID: 19230772
-
List A et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456-1465. PMID: 17021321
-
Platzbecker U et al. ELN MDS recommendations. Blood. 2021;138(22):2165-2168. PMID: 34773327
Viva Points
"MDS are clonal stem cell disorders with dysplasia, cytopenias, and AML risk. Diagnosis requires bone marrow examination. Stratify using IPSS-R. Lower-risk: supportive care, ESAs, lenalidomide for del(5q). Higher-risk: azacitidine, allo-SCT."
Key Facts
- Bone marrow dysplasia ≥10% in one lineage
- IPSS-R for prognosis and treatment decisions
- del(5q): lenalidomide responsive
- SF3B1 mutation: ring sideroblasts, favourable
- TP53 mutation: very poor prognosis
- Azacitidine: standard for higher-risk
Common Mistakes
- ❌ Diagnosing MDS without bone marrow
- ❌ Not excluding B12/folate deficiency first
- ❌ Not calculating IPSS-R
- ❌ Treating higher-risk as lower-risk
Last Reviewed: 2026-01-01 | MedVellum Editorial Team