Drug-Induced Liver Injury
Summary
Drug-induced liver injury (DILI) is a leading cause of acute liver failure and drug withdrawal from the market. It encompasses a spectrum from asymptomatic transaminase elevation to fulminant hepatic failure. DILI can be classified as intrinsic (dose-dependent, predictable - e.g., paracetamol) or idiosyncratic (unpredictable, not strictly dose-related - e.g., amoxicillin-clavulanate). Pattern classification includes hepatocellular (ALT predominant), cholestatic (ALP predominant), or mixed. The R-value (ALT/ULN ÷ ALP/ULN) determines pattern. Hy's Law identifies cases with high mortality risk: hepatocellular injury with jaundice carries 10% mortality. Management is primarily drug withdrawal and supportive care.
Key Facts
- Definition: Liver injury caused by medications, herbal products, or dietary supplements
- Incidence: 14-19 per 100,000 persons annually; most common cause of ALF in Western countries
- Mortality: Hy's Law cases 10% mortality; overall DILI 5-10%
- Common Drugs: Paracetamol (intrinsic), amoxicillin-clavulanate, isoniazid, NSAIDs, statins, antiepileptics
- Pathognomonic: Temporal relationship between drug exposure and liver injury; resolution after discontinuation
- Gold Standard: Clinical diagnosis of exclusion + RUCAM causality score
- First-line Treatment: Stop offending drug; NAC for paracetamol; supportive care
- Prognosis: Most resolve; ALF develops in 1-5%
Clinical Pearls
Diagnostic Pearl: Calculate R-value: (ALT/ULN) ÷ (ALP/ULN). R≥5 = hepatocellular; R≤2 = cholestatic; R 2-5 = mixed.
Emergency Pearl: Hy's Law: ALT >3x ULN + total bilirubin >2x ULN + absence of cholestasis = ~10% mortality risk. Urgent hepatology referral.
Treatment Pearl: N-acetylcysteine has benefit even in non-paracetamol ALF and should be considered for severe DILI.
Pitfall Warning: Herbal and dietary supplements cause 20% of DILI - always take full medication history including supplements.
Mnemonic: DILI STOP - Drug history complete, Investigations (exclude other causes), Liver pattern (R-value), Identify high-risk (Hy's Law), Stop offending drug, Time to recovery, Other support, Paracetamol protocol if applicable
Why This Matters Clinically
DILI is the most common cause of acute liver failure in the UK/US and a leading reason for post-marketing drug withdrawal. Early recognition prevents progression to ALF. This is a core patient safety and pharmacovigilance topic relevant to all prescribing clinicians.
Incidence and Prevalence
- Incidence: 14-19 per 100,000 population per year
- ALF from DILI: 50% of ALF cases in Western countries (paracetamol dominant)
- Idiosyncratic DILI: 1-10 per 100,000 drug exposures depending on drug
Common Causative Agents
| Drug Class | Examples | Pattern |
|---|---|---|
| Antimicrobials | Amoxicillin-clavulanate, flucloxacillin, isoniazid | Cholestatic/Mixed |
| Analgesics | Paracetamol (intrinsic), diclofenac, sulindac | Hepatocellular |
| Anticonvulsants | Phenytoin, valproate, carbamazepine | Hepatocellular |
| Cardiovascular | Amiodarone, statins, methyldopa | Variable |
| Psychiatric | Chlorpromazine, risperidone | Cholestatic |
| Supplements | Green tea extract, kava, black cohosh | Hepatocellular |
Risk Factors
| Factor | Impact |
|---|---|
| Age >55 years | Higher risk and severity |
| Female sex | 2x risk for idiosyncratic DILI |
| Pre-existing liver disease | Increased susceptibility |
| Polypharmacy | Drug interactions |
| Alcohol use | Synergistic hepatotoxicity |
| Genetic polymorphisms | HLA associations (e.g., flucloxacillin-HLA-B*57:01) |
Mechanism
Intrinsic (Dose-Dependent) DILI:
- Direct hepatotoxin or toxic metabolite
- Example: Paracetamol → NAPQI (via CYP2E1) → glutathione depletion → hepatocyte death
- Predictable, dose-related
Idiosyncratic DILI:
- Unpredictable, not strictly dose-dependent
- Mechanisms: immune-mediated (hapten formation, HLA-restricted), metabolic idiosyncrasy
- Variable latency (days to months)
Step 1: Drug/Metabolite Formation
- Parent drug or metabolite (via CYP450) causes injury
- Reactive metabolites bind cellular proteins
Step 2: Hepatocyte Stress/Death
- Oxidative stress, mitochondrial dysfunction
- Apoptosis or necrosis of hepatocytes
- Release of DAMPs
Step 3: Immune Activation (Idiosyncratic)
- Drug-protein adducts as neoantigens
- T-cell mediated response
- May have eosinophilia, rash (DRESS-like)
Step 4: Liver Injury Pattern
- Hepatocellular: Zone 3 necrosis (paracetamol), diffuse hepatitis
- Cholestatic: Bile duct injury, canalicular transport inhibition
- Mixed: Features of both
Step 5: Recovery or Progression
- Drug discontinuation usually leads to recovery
- Severe cases progress to ALF requiring transplant
Classification by Pattern
| Pattern | R-Value | Features |
|---|---|---|
| Hepatocellular | R ≥5 | ALT predominant; higher ALF risk |
| Cholestatic | R ≤2 | ALP predominant; jaundice, pruritus |
| Mixed | R 2-5 | Features of both |
R = (ALT/ULN) ÷ (ALP/ULN)
Symptoms
Acute:
Cholestatic:
Severe/ALF:
Signs
Red Flags
[!CAUTION] Hy's Law - High mortality marker:
- ALT or AST >3x ULN
- AND bilirubin >2x ULN
- AND no cholestatic pattern (ALP less than 2x)
Also: INR >1.5, encephalopathy, hypoglycaemia
Assessment
General:
- Jaundice assessment
- Mental status (encephalopathy grading)
- Signs of chronic liver disease (absent in acute)
Abdominal:
- RUQ tenderness
- Hepatomegaly
- Ascites (suggests severe/chronic)
Skin:
- Rash (drug hypersensitivity)
- Bruising (coagulopathy)
First-Line
- LFTs: ALT, AST, ALP, bilirubin
- INR: Synthetic function
- Paracetamol level (if indicated)
Laboratory
| Test | Purpose |
|---|---|
| ALT, AST | Hepatocyte injury |
| ALP, GGT | Cholestasis |
| Bilirubin | Severity, Hy's Law |
| INR | Synthetic function |
| Albumin | Chronic/severe liver disease |
| FBC | Eosinophilia (hypersensitivity) |
| Paracetamol level | Specific antidote available |
| Viral hepatitis serology | Exclude HBV, HAV, HCV, HEV |
| Autoimmune markers | ANA, SMA, IgG (exclude AIH) |
| Caeruloplasmin | Wilson disease if less than 40 years |
Imaging
| Modality | Purpose |
|---|---|
| USS Abdomen | Exclude biliary obstruction, Budd-Chiari |
| CT/MRI | If structural lesion suspected |
Causality Assessment (RUCAM Score)
Roussel Uclaf Causality Assessment Method:
- Time to onset
- Course after stopping drug
- Risk factors
- Concomitant drugs
- Exclusion of other causes
- Re-challenge (if performed)
Score interpretation:
- ≤0: Excluded
- 1-2: Unlikely
- 3-5: Possible
- 6-8: Probable
- ≥9: Highly probable
Algorithm
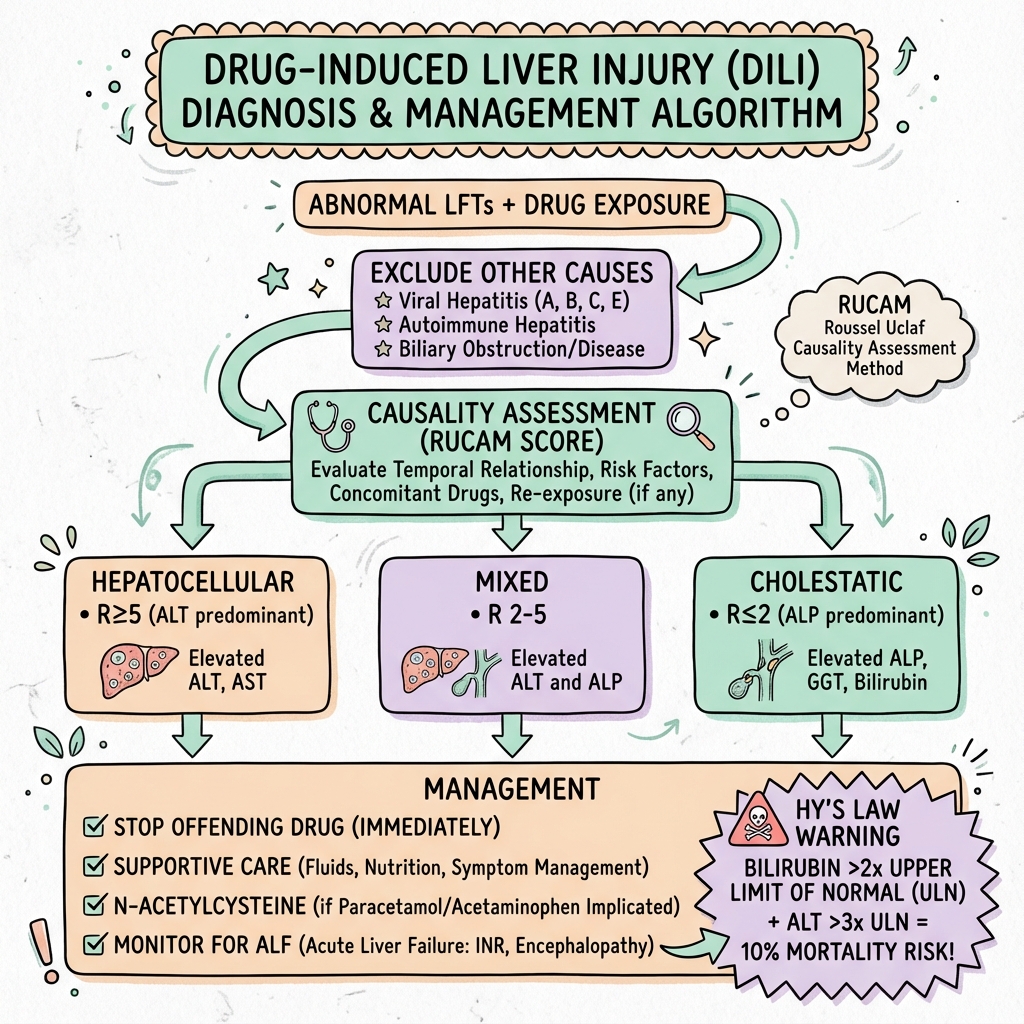
Immediate Management
- Stop suspected drug(s) immediately
- Check paracetamol level and treat if indicated
- Assess severity: INR, encephalopathy, Hy's Law
- Exclude other causes of liver injury
- Urgent hepatology referral if ALF features
Specific Treatment
Paracetamol Overdose:
- N-acetylcysteine (NAC) per nomogram
- NAC also beneficial in non-paracetamol ALF
Carnitine for Valproate Toxicity:
- L-carnitine IV if valproate-induced hepatotoxicity
Immunosuppression:
- Corticosteroids for severe hypersensitivity/DRESS-DILI (controversial)
Supportive Care
- Monitor closely: LFTs, INR, glucose, renal function
- Nutritional support
- Avoid hepatotoxins
- ICU if ALF features
- Liver transplant evaluation if ALF
Drug Rechallenge
- Generally avoided
- May be considered if drug essential and DILI was mild
- Contraindicated if: Hy's Law, hypersensitivity features, severe injury
Disposition
- Admit: Hy's Law, INR >1.5, encephalopathy, bilirubin >100
- Outpatient: Mild asymptomatic transaminase elevation
- Transplant centre: ALF features
| Complication | Incidence | Management |
|---|---|---|
| Acute liver failure | 1-5% | Transplant evaluation |
| Chronic liver disease | 5-10% | Monitoring, may resolve |
| Cholestatic syndrome | 10% | Ursodeoxycholic acid |
| Death | 5-10% overall; 10% Hy's Law | Prevention, early recognition |
Outcomes
- Most DILI resolves within 1-3 months after drug cessation
- Hy's Law cases: 10% mortality without transplant
- ALF requiring transplant: 50% survival
- Chronic injury develops in 5-10%
Prognostic Indicators
Good:
- Rapid drug cessation
- Cholestatic pattern (lower mortality than hepatocellular)
- Mild elevation (ALT less than 5x)
- No Hy's Law criteria
Poor:
- Hy's Law positive
- Hepatocellular pattern with jaundice
- INR >1.5
- Encephalopathy
- Delayed diagnosis
Key Guidelines
- EASL Clinical Practice Guidelines: DILI (2019) — Comprehensive European guidance PMID: 30926241
- ACG Clinical Guideline: DILI (2021) — American College of Gastroenterology PMID: 33929376
Key Studies
- DILIN Registry — Largest prospective DILI registry, characterising patterns and outcomes
- Hy's Law — FDA guidance on hepatotoxicity signal in drug development
What is DILI?
Some medications can cause liver damage. This can happen with almost any medication, including herbal supplements.
How serious is it?
Usually mild and recovers when the medication is stopped. Rarely, it can cause serious liver failure needing hospital treatment.
What should you do?
Tell your doctor about all medications and supplements you take. If you develop yellowing skin, dark urine, or severe tiredness after starting a new medication, seek medical attention promptly.
-
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70(6):1222-1261. PMID: 30926241
-
Chalasani NP et al. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am J Gastroenterol. 2021;116(5):878-898. PMID: 33929376
-
Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury. Int J Mol Sci. 2016;17(1):14. PMID: 26712732
-
Zimmerman HJ. The spectrum of hepatotoxicity. Perspect Biol Med. 1968;12(1):135-161. PMID: 4884878
-
Reuben A et al. Drug-induced acute liver failure: Results of a US multicenter, prospective study. Hepatology. 2010;52(6):2065-2076. PMID: 20949552
Viva Points
"DILI is liver injury caused by medications or supplements. Classified by pattern using R-value: hepatocellular (R≥5), cholestatic (R≤2), mixed. Hy's Law (ALT >3x + bilirubin >2x + no cholestasis) indicates 10% mortality. Management: stop drug, NAC if paracetamol, supportive care, hepatology referral if severe."
Key Facts
- R-value calculation: (ALT/ULN) ÷ (ALP/ULN)
- Hy's Law: 10% mortality marker
- Amoxicillin-clavulanate most common cause idiosyncratic DILI
- RUCAM score for causality assessment
Common Mistakes
- ❌ Forgetting herbal/supplement history
- ❌ Not calculating R-value
- ❌ Missing Hy's Law cases
- ❌ Delayed drug cessation
Last Reviewed: 2026-01-01 | MedVellum Editorial Team